1. Valtoco. Prescribing Information. Neurelis Inc; 2023; 2. Data on file. REF-01245. Neurelis Inc; 3. Data on file. REF-01288. Neurelis Inc; 4. Wheless JW, Miller I, Hogan RE, et al; DIAZ.001.05 Study Group. Final results from a Phase 3, long-term, open-label, repeat-dose safety study of diazepam nasal spray for seizure clusters in patients with epilepsy. Epilepsia. 2021;62(10):2485-2495. doi:10.1111/epi.17041; 5. Tarquinio D, Dlugos D, Wheless JW, et al. Safety of Valtoco® (diazepam nasal spray) in children and adolescents with epilepsy: final subgroup results from a phase 3, long-term, open-label, repeat-dose safety study. Poster presented at: Child Neurology Society 50th Annual Meeting; September 29 – October 2, 2021; Boston, MA; 6. Sperling MR, Wheless JW, Hogan RE, et al; DIAZ 001.05 Study Group. Use of second doses of Valtoco® (diazepam nasal spray) across 24 hours after the initial dose for out-of-hospital seizure clusters: results from a phase 3, open-label, repeat-dose safety study. Epilepsia. 2022;63(4):836-843. doi:10.1111/epi.17177; 7. Data on file. REF-01292. Neurelis Inc; 8. Penovich P, Wheless JW, Hogan RE, et al. Examining the patient and caregiver experience with diazepam nasal spray for seizure clusters: results from an exit survey of a phase 3, open-label, repeat-dose safety study. Epilepsy Behav. 2021;121(pt A):108013. doi:10.1016/j.yebeh.2021.108013; 9. Misra SN, Sperling MR, Rao VR, et al. Significant improvements in SEIzure interVAL (time between seizure clusters) across time in patients treated with diazepam nasal spray as intermittent rescue therapy for seizure clusters. Epilepsia. 2022;63(10):2684-2693. doi:10.1111/epi.17385; 10. Hogan RE, Tarquinio D, Sperling MR, et al. Pharmacokinetics and safety of VALTOCO (NRL-1; diazepam nasal spray) in patients with epilepsy during seizure (ictal/peri-ictal) and nonseizure (interictal) conditions: a phase 1, open-label study. Epilepsia. 2020;61(5):935-943. doi:10.1111/epi.16506; 11. Agarwal SK, Kriel RL, Brundage RC, Ivaturi VD, Cloyd JC. A pilot study assessing the bioavailability and pharmacokinetics of diazepam after intranasal and intravenous administration in healthy volunteers. Epilepsy Res. 2013;105(3):362-367. doi:10.1016/j.eplepsyres.2013.02.018; 12. Tanimoto S, Koplowitz LP, Lowenthal RE, Koplowitz B, Rabinowicz AL, Carrazana E. Evaluation of pharmacokinetics and dose proportionality of diazepam after intranasal administration of NRL-1 to healthy volunteers. Clin Pharmacol Drug Dev. 2020;9(6):719-727. doi:10.1002/cpdd.767; 13. Rabinowicz AL, Carrazana E, Maggio ET. Improvement of intranasal drug delivery with Intravail® alkylsaccharide excipient as a mucosal absorption enhancer aiding in the treatment of conditions of the central nervous system. Drugs R D. 2021;21(4):361-369. doi:10.1007/s40268-021-00360-5; 14. Milligan N, Dhillon S, Richens A, Oxley J. Rectal diazepam in the treatment of absence status: a pharmacodynamic study. J Neurol Neurosurg Psychiatry. 1981;44(10):914-917. doi:10.1136/jnnp.44.10.914; 15. Dhir A, Rogawski MA. Determination of minimal steady-state plasma level of diazepam causing seizure threshold elevation in rats. Epilepsia. 2018;59(5):935-944. doi:10.1111/epi.14069; 16. Hogan RE, Gidal BE, Koplowitz B, Koplowitz LP, Lowenthal RE, Carrazana E. Bioavailability and safety of diazepam intranasal solution compared to oral and rectal diazepam in healthy volunteers. Epilepsia. 2020;61(3):455-464. doi:10.1111/epi.16449; 17. Clinical superiority findings: 2019: Neurelis Pharmaceuticals, Inc. for Valtoco (diazepam nasal spray). US Food and Drug Administration. Accessed September 10, 2023. https://www.fda.gov/industry/designating-orphan-product-drugs-and-biological-products/clinical-superiority-findings; 18. Misra SN, Faught E, Davis C, Carrazana E, Rabinowicz AL. Results for the Quality of Life in Epilepsy scale from a long-term safety study of diazepam nasal spray for seizure clusters. Poster presented at: American Epilepsy Society 75th Annual Meeting; December 3-7, 2021; Chicago, IL.
VALTOCO offers rapid, reliable, ready seizure rescue VALTOCO offers rapid, reliable, ready
seizure rescue
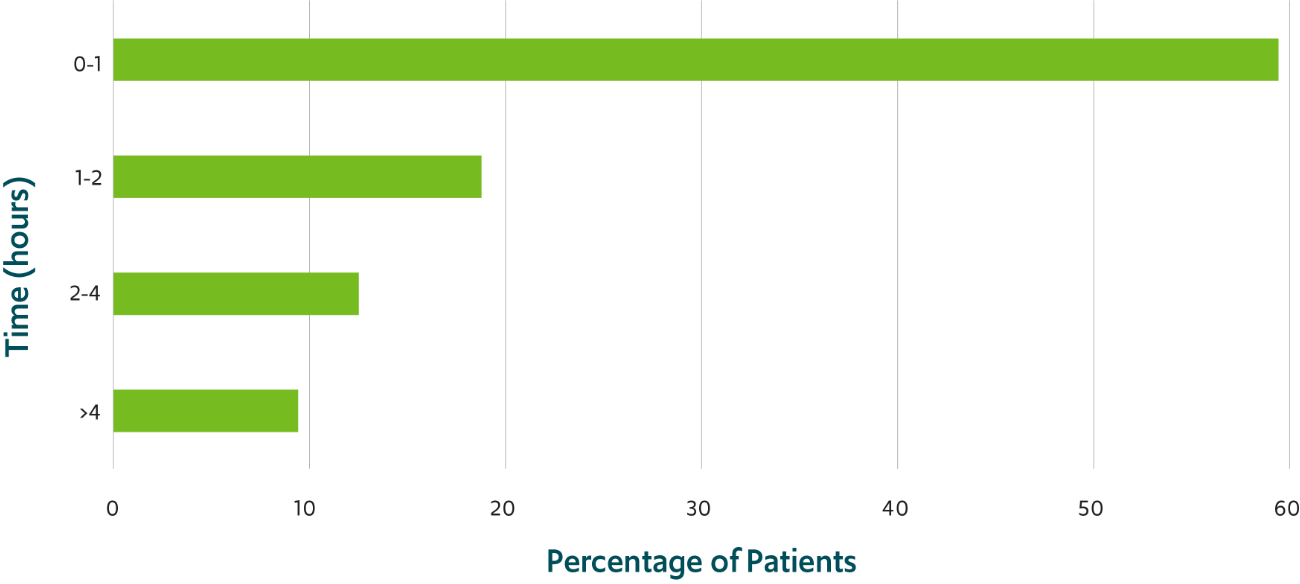
A majority of patients returned to their usual selves within 1 hour of administration of VALTOCO8,‡
Treatment-related somnolence was reported in 1.8% of patients4
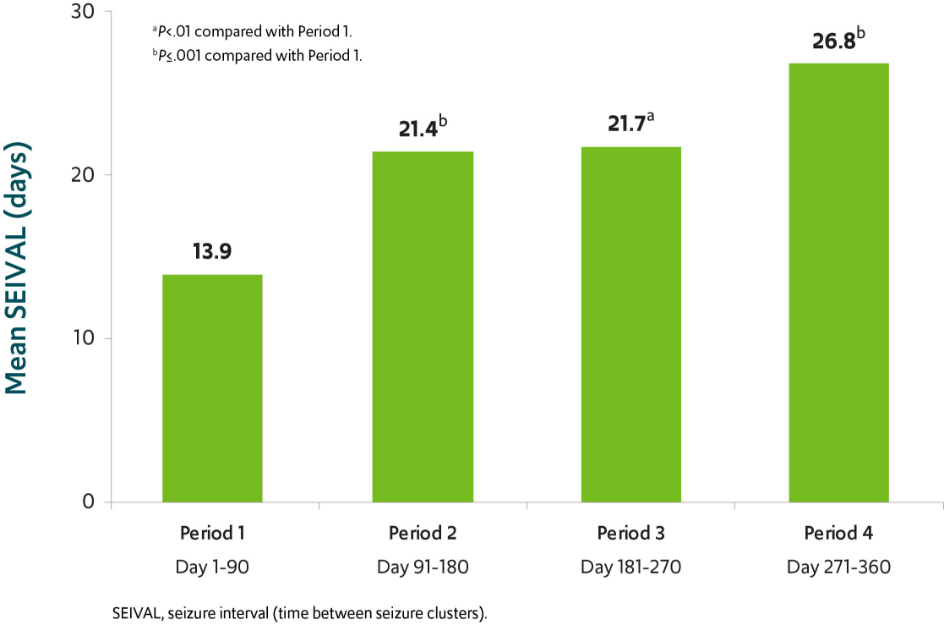
The number of seizure-free days between treated seizure clusters doubled over a 12-month period in patients using VALTOCO9,§
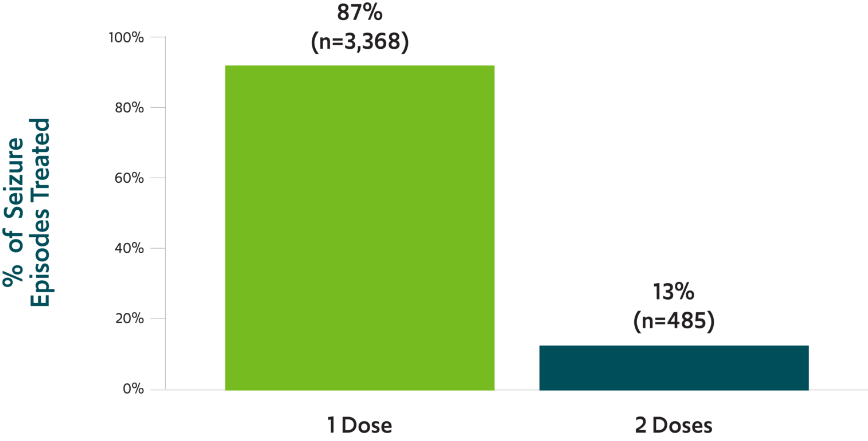
*This was an exploratory analysis; the study did not have prespecified efficacy endpoints.4
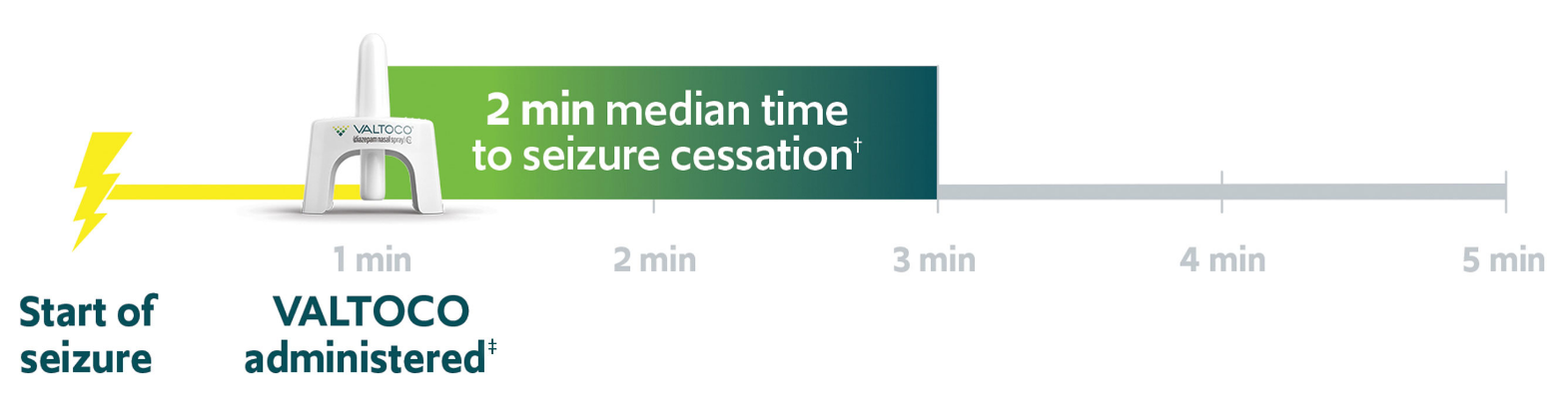
†Median time to seizure cessation of 4 minutes (range, -1-1151 minutes).2
‡59% of patients returned to their usual selves within 60 minutes of administration of VALTOCO as recorded in patient and caregiver surveys.8
§SEIVAL was measured as the time between VALTOCO doses using seizure diary data from the open-label safety study. Seventy-six of the 163 patients (47%) who received >_1 dose of VALTOCO in the study had >_1 SEIVAL in each of Periods 1, 2, 3, and 4 and were included in the consistent cohort for Periods 1-4. Retreatments, defined as second doses given within 24 hours of the first doses, were eliminated from the analysis.9

Only VALTOCO uses Intravail® technology for rapid and reliable absorption of diazepam11,12
VALTOCO was formulated to address the principal limitations of intranasal drug delivery. Intravail® technology facilitates increased drug absorption across the nasal mucosa by increasing permeability of cell membranes and temporarily loosening tight junctions between cells, allowing the drug to pass through.13
Nearly complete absorption of VALTOCO with 97% bioavailability relative
to intravenous diazepam1,11
Therapeutic plasma levels of VALTOCO sustained for 24 hours 1,11
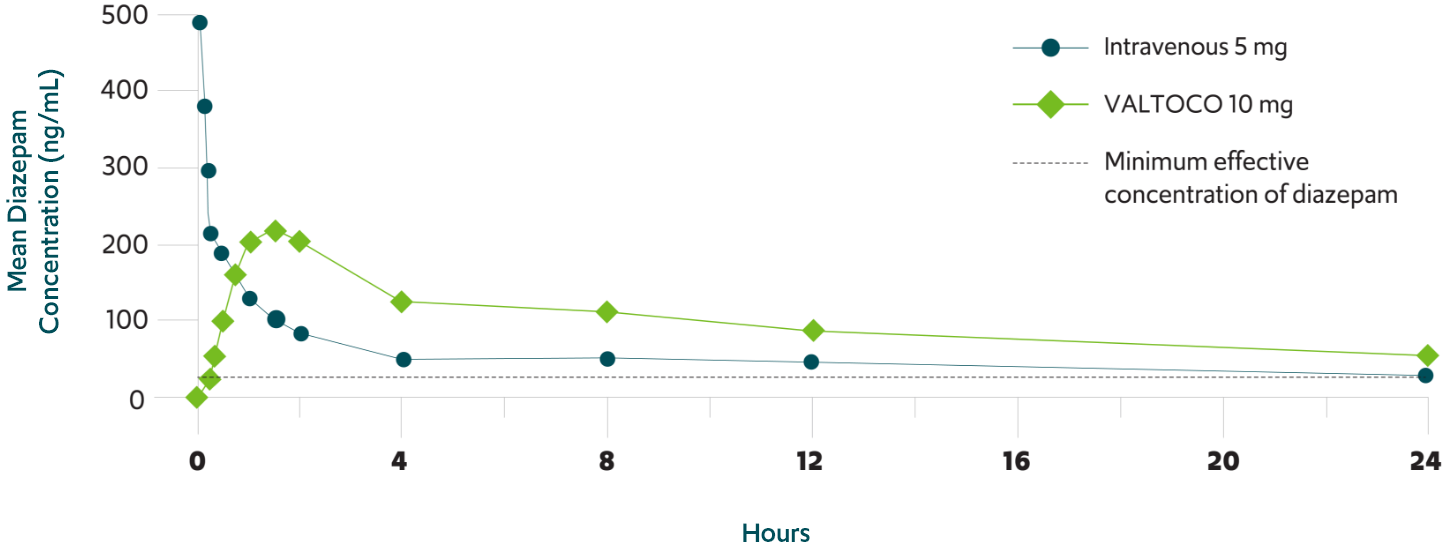
Reduction in seizure activity can occur at a diazepam concentration as low as 30 ± 15 ng/mL14,15
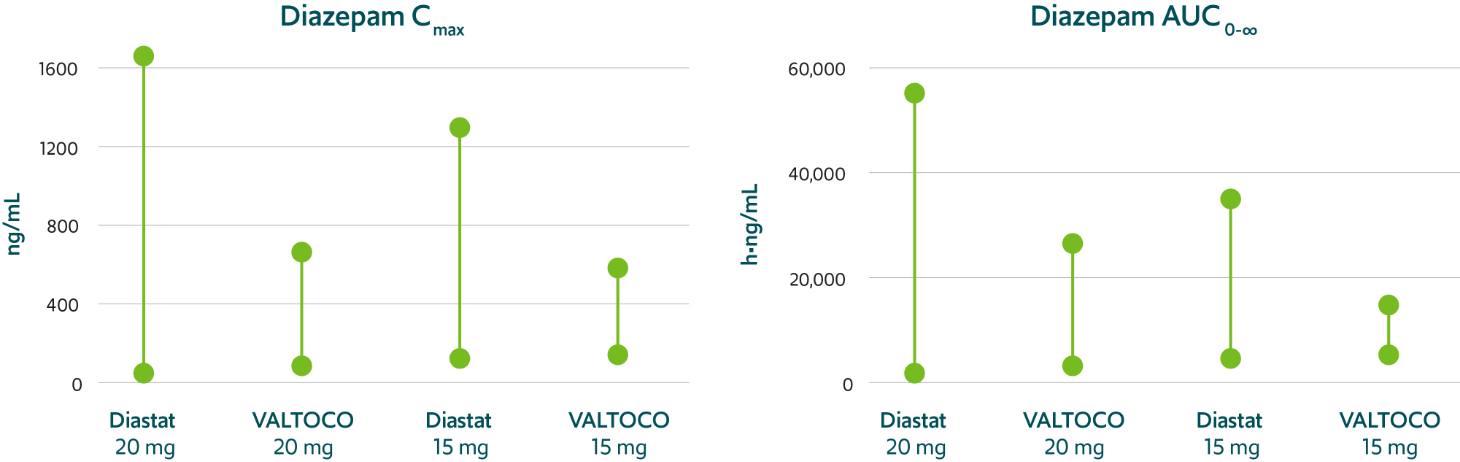
Predictable pharmacokinetics with low interpatient and intrapatient variability1,12,16
VALTOCO demonstrated 2- to 4-fold less pharmacokinetic variability than Diastat® (diazepam rectal gel) regardless
of patient weight1,12,16
Pharmacokinetic variability profiles of VALTOCO and Diastat 16
AUC, area under the curve; Cmax, maximum serum concentration.
90% CI
VALTOCO pharmacokinetics were not notably altered when administered during seizures vs under normal conditions10
See how VALTOCO works to optimize diazepam nasal delivery
Learn how the unique formulation of VALTOCO optimizes diazepam for nasal delivery, and how it helps patients manage episodes of frequent seizure activity whenever, wherever.

VALTOCO was deemed clinically superior in administration to Diastat® (diazepam rectal gel) by the US Food and Drug Administration (FDA)17
The intranasal route of administration of VALTOCO provides a major contribution to patient care over the rectal
route of administration by providing a significantly improved ease of use.17
Because of this, the FDA has determined that VALTOCO deserves orphan drug exclusivity.17
VALTOCO was strongly preferred in
a patient survey8,*
*Data collected from patient diaries and patient and care partner surveys.8
90% said it was “extremely easy” or “very easy” to train others to administer VALTOCO
87% felt more comfortable being treated in public with VALTOCO than with diazepam rectal gel
84% said they would prefer using VALTOCO as their seizure rescue exclusively in the future
79% were very comfortable doing activities outside the home with VALTOCO available
Improvements in seizure worry and social functioning18
PATIENTS WITH EPILEPSY

Mean total QOLIE-31-P scores increased from day 0 (n=71) to day 365 (n=53) by 5.2 points (from 57.3 to 62.5), which also exceeded the MIC threshold of 5.19.
MIC, minimally important change; QOLIE-31-P, Quality of Life in Epilepsy—Problems.
Help your patients get to know VALTOCO
Get all the resources you need so you can educate your patients about VALTOCO.
Get VALTOCO Resources
Indication
VALTOCO® (diazepam nasal spray) is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures) that are distinct from a patient’s usual seizure pattern in patients with epilepsy 6 years of age and older.
IMPORTANT SAFETY INFORMATION
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation.
The use of benzodiazepines, including VALTOCO, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing VALTOCO and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction.
The continued use of benzodiazepines may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Although VALTOCO is indicated only for intermittent use, if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction of VALTOCO may precipitate acute withdrawal reactions, which can be life-threatening. For patients using VALTOCO more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue VALTOCO.
Contraindications: VALTOCO is contraindicated in patients with:
Hypersensitivity to diazepam
Acute narrow-angle glaucoma
Central Nervous System (CNS) Depression
Benzodiazepines, including VALTOCO, may produce CNS depression. Caution patients against engaging in hazardous activities requiring mental alertness, such as operating machinery, driving a motor vehicle, or riding a bicycle, until the effects of the drug, such as drowsiness, have subsided, and as their medical condition permits.
The potential for a synergistic CNS-depressant effect when VALTOCO is used with alcohol or other CNS depressants must be considered, and appropriate recommendations made to the patient and/or care partner.
Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including VALTOCO, increase the risk of suicidal ideation and behavior. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or unusual changes in mood or behavior.
Glaucoma
Benzodiazepines, including VALTOCO, can increase intraocular pressure in patients with glaucoma. VALTOCO may be used in patients with open-angle glaucoma only if they are receiving appropriate therapy. VALTOCO is contraindicated in patients with narrow-angle glaucoma.
Neonatal Sedation and Withdrawal Syndrome
Use of VALTOCO late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate. Monitor neonates exposed to VALTOCO during pregnancy or labor for signs of sedation and monitor neonates exposed to VALTOCO during pregnancy for signs of withdrawal; manage these neonates accordingly.
Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preservative
VALTOCO is not approved for use in neonates or infants. Serious and fatal adverse reactions, including “gasping syndrome,” can occur in neonates and low-birth-weight infants treated with benzyl alcohol–preserved drugs, including VALTOCO. The “gasping syndrome” is characterized by central nervous system depression, metabolic acidosis, and gasping respirations. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known.
Adverse Reactions
The most common adverse reactions (at least 4%) were somnolence, headache, and nasal discomfort.
Diazepam, the active ingredient in VALTOCO, is a Schedule IV controlled substance.
To report SUSPECTED ADVERSE REACTIONS, contact Neurelis, Inc. at 1-866-696-3873 or FDA at 1-800-FDA-1088 (www.fda.gov/medwatch).
Please see full Prescribing Information, including Boxed Warning.
IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNING

Indication
VALTOCO® (diazepam nasal spray) is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures) that are distinct from a patient’s usual seizure pattern in patients with epilepsy 6 years of age and older.
IMPORTANT SAFETY INFORMATION
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation.
The use of benzodiazepines, including VALTOCO, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing VALTOCO and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction.
The continued use of benzodiazepines may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Although VALTOCO is indicated only for intermittent use, if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction of VALTOCO may precipitate acute withdrawal reactions, which can be life-threatening. For patients using VALTOCO more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue VALTOCO.
Contraindications: VALTOCO is contraindicated in patients with:
Hypersensitivity to diazepam
Acute narrow-angle glaucoma
Central Nervous System (CNS) Depression
Benzodiazepines, including VALTOCO, may produce CNS depression. Caution patients against engaging in hazardous activities requiring mental alertness, such as operating machinery, driving a motor vehicle, or riding a bicycle, until the effects of the drug, such as drowsiness, have subsided, and as their medical condition permits.
The potential for a synergistic CNS-depressant effect when VALTOCO is used with alcohol or other CNS depressants must be considered, and appropriate recommendations made to the patient and/or care partner.
Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including VALTOCO, increase the risk of suicidal ideation and behavior. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or unusual changes in mood or behavior.
Glaucoma
Benzodiazepines, including VALTOCO, can increase intraocular pressure in patients with glaucoma. VALTOCO may be used in patients with open-angle glaucoma only if they are receiving appropriate therapy. VALTOCO is contraindicated in patients with narrow-angle glaucoma.
Neonatal Sedation and Withdrawal Syndrome
Use of VALTOCO late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate. Monitor neonates exposed to VALTOCO during pregnancy or labor for signs of sedation and monitor neonates exposed to VALTOCO during pregnancy for signs of withdrawal; manage these neonates accordingly.
Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preservative
VALTOCO is not approved for use in neonates or infants. Serious and fatal adverse reactions, including “gasping syndrome,” can occur in neonates and low-birth-weight infants treated with benzyl alcohol–preserved drugs, including VALTOCO. The “gasping syndrome” is characterized by central nervous system depression, metabolic acidosis, and gasping respirations. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known.
Adverse Reactions
The most common adverse reactions (at least 4%) were somnolence, headache, and nasal discomfort.
Diazepam, the active ingredient in VALTOCO, is a Schedule IV controlled substance.
To report SUSPECTED ADVERSE REACTIONS, contact Neurelis, Inc. at 1-866-696-3873 or FDA at 1-800-FDA-1088 (www.fda.gov/medwatch).
Please see full Prescribing Information, including Boxed Warning.
References

Get Copay Card

Order Demo Kit