VALTOCO safety profile
VALTOCO offers established safety and tolerability
In a 12-month, open-label, repeat-dose safety study in patients 6 to 65 years of age:
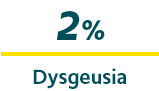
The most common treatment-related adverse events (AEs), defined as those occurring in ≥2% of patients, were1,*:
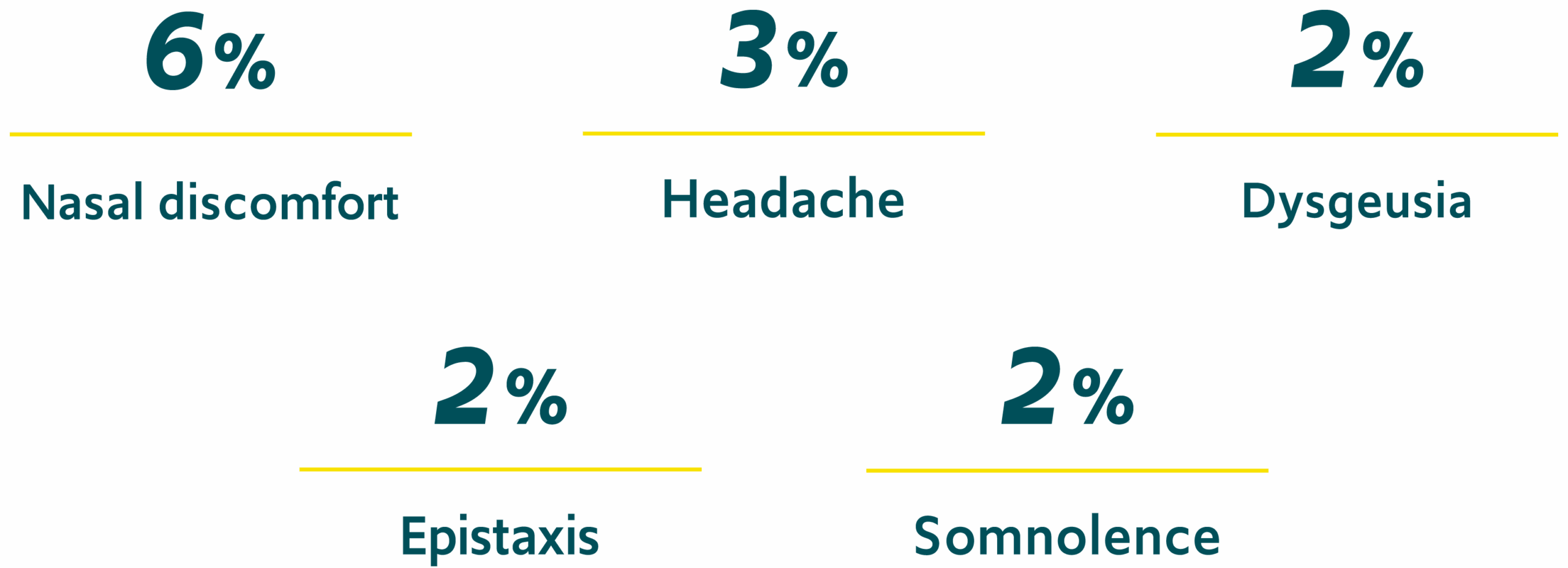
No clinically relevant changes in respiratory rate were reported.1,*
A consistent safety and tolerability profile in patients 2 to 5 years of age
There were 8 treatment-related AEs in 7 patients, including2,3,†:
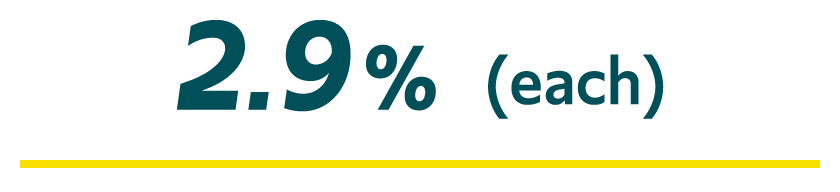
Nasal edema, vomiting, nasal mucosal disorder, rhinorrhea, blepharitis, somnolence, administration site pain, and aspiration pneumonia (n=1 each)
Respiratory depression, respiratory distress, and hypoxia were reported by 1 patient each; none were attributed to treatment.2,†
*Open-label, repeat-dose safety study enrolling 175 patients with epilepsy 6 to 65 years of age; there were 3,853 seizure episodes in 163 patients who received ≥1 dose of VALTOCO.1
†Open-label, pharmacokinetic (PK) and repeat-dose safety study in patients with epilepsy 2 to 5 years of age; 35 patients were enrolled and received ≥1 dose of VALTOCO (35 PK assessments and 227 seizure episodes).2,3