Prepare your patients to stop seizures in their tracks
VALTOCO is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures) that are distinct from a patient’s usual seizure pattern in patients with epilepsy 2 years of age and older.1
Prepare your patients to stop seizures in their tracks
VALTOCO is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures) that are distinct from a patient’s usual seizure pattern in patients with epilepsy 2 years of age and older.1
In open-label safety studies:

The median time to seizure cessation when VALTOCO was administered within 5 minutes of seizure onset was 2 minutes2,*,†
VALTOCO is most effective when administered early*

2-minute median time to seizure cessation when VALTOCO was administered within 5 minutes of seizure onset2
(n=2256 seizures; range, 0-1440 minutes)
The median time to seizure cessation for all seizures treated with VALTOCO regardless of time to administration was 3 minutes (n=3444; range, 0-1440 minutes).2

87% of seizure episodes used a single dose over a 24-hour period3,*,‡
Use of single doses of VALTOCO over 24 hours
Percentage of seizure episodes for which a single dose of VALTOCO was used over the course of 24 hours (N=4080)3,‡
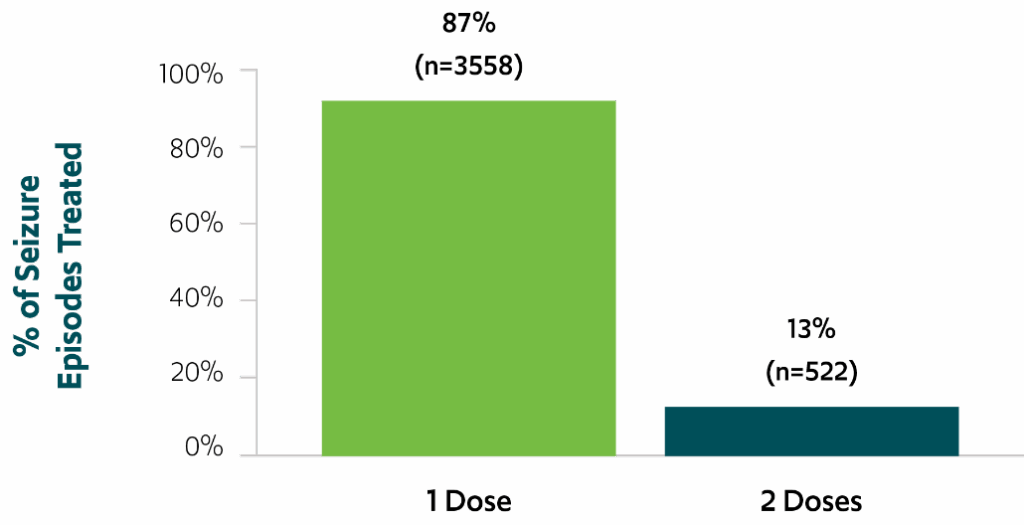

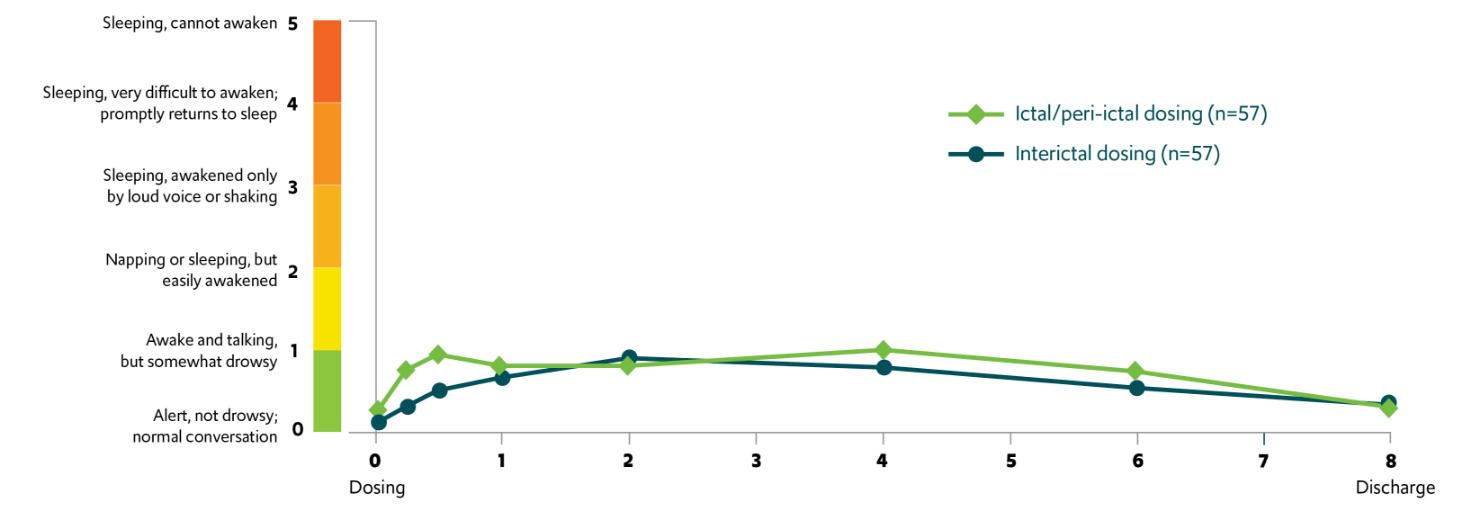
A majority of patients aged ≥6 years returned to their usual selves within 1 hour of VALTOCO administration4,§,‖
Across long-term, open-label safety studies, treatment-related somnolence never resulted in treatment discontinuation.5,6
Return to self and mean sedation levels
In another study, overall mean sedation levels were low, mild, transient, and not considered clinically relevant7,#
Analysis of time between treated episodes of frequent seizures**
Patients with Epilepsy
The number of days between treated episodes of frequent seizures nearly doubled in patients using VALTOCO over a 12-month period8
SEIVAL increased by 12.9 days over a 12-month period (n=76)8
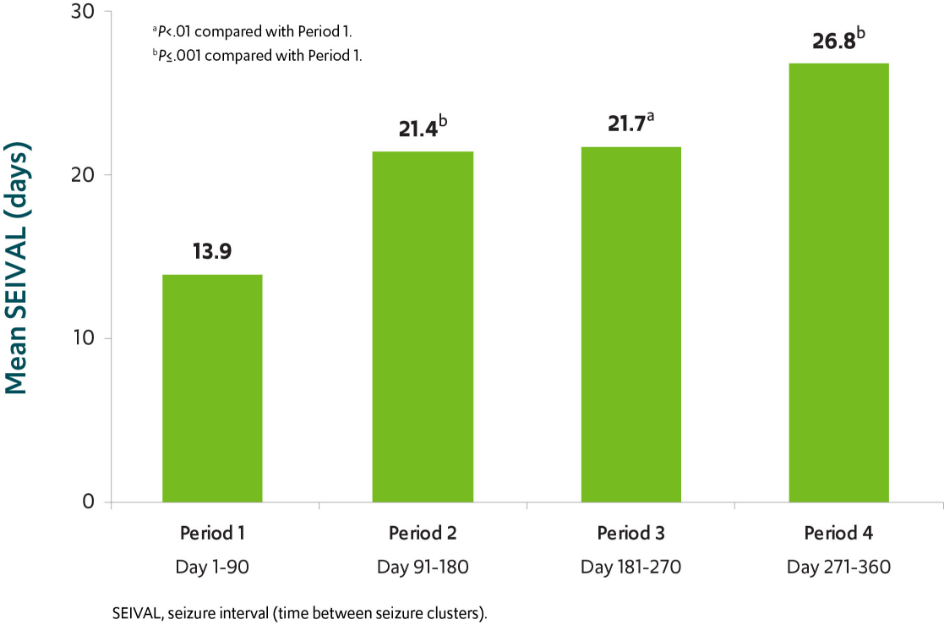
VALTOCO was shown to prolong the interval between treated episodes of frequent seizures and therefore decrease the frequency of those episodes.9,10
Patients with Epilepsy
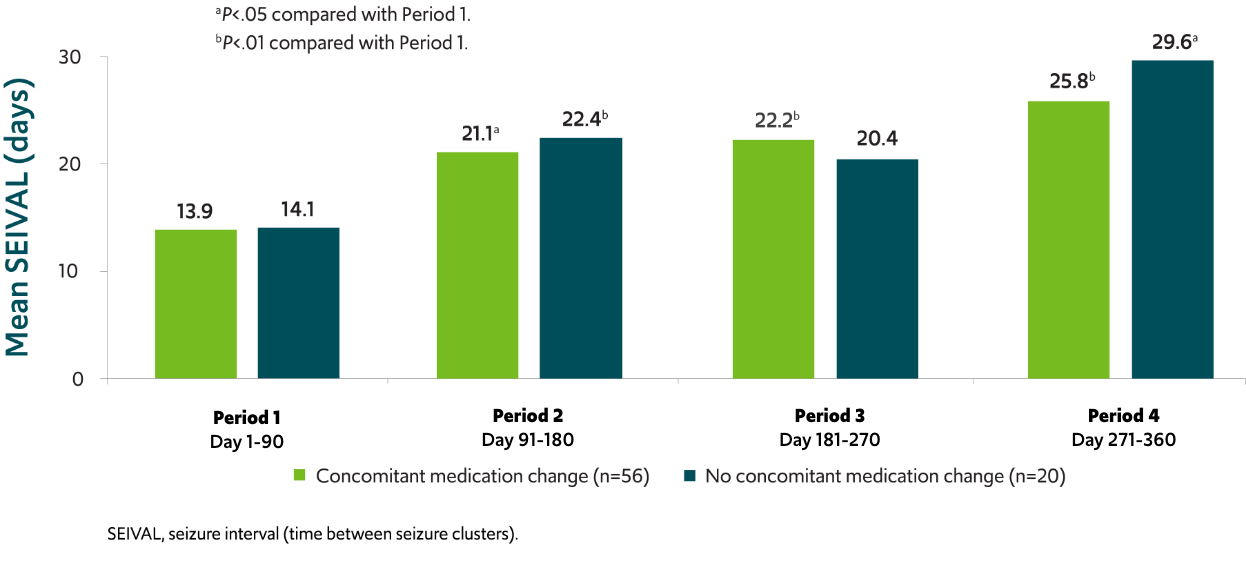
SEIVAL increased independent of concomitant medication change8
*Post hoc analyses from Study 05, a 12-month, open-label, repeat-dose safety study in patients 6 to 65 years of age, and Study 08, an open-label, pharmacokinetic and repeat-dose safety study in patients 2 to 5 years of age; the studies did not have prespecified efficacy endpoints.5,6
†All data represented as median values. Missing date/time points, seizures lasting >24 hours, seizures in which VALTOCO was administered prior to seizure start time, and seizures stopping prior to seizure starts were excluded from the analysis.11
‡Per VALTOCO Prescribing Information, a second dose may be given at least 4 hours after the initial dose, if required. Post hoc analyses of 163 patients from Study 05, a 12-month, open-label, repeat-dose safety study in patients 6 to 65 years of age, and 35 patients from Study 08, an open-label, pharmacokinetic and repeat-dose safety study in patients 2 to 5 years of age; the studies did not have prespecified efficacy endpoints.1,5,6
§59% of patients 6 to 65 years of age returned to their usual selves within 60 minutes of administration of VALTOCO as recorded in patient and caregiver surveys.4
‖Analysis from Study 05 (N=163), a 12-month, open-label, repeat-dose safety study in patients 6 to 65 years of age; the study did not have prespecified efficacy endpoints.5
#A phase 1, open-label study assessed the pharmacokinetics and safety of VALTOCO in patients with epilepsy (N=57) 6 to 59 years of age during seizure and nonseizure states. VALTOCO was administered to each patient during 2 periods (≥14-day washout between). Sedation was assessed using a 6-point scale. Sedation scores indicated small increases in sedation overall. These small increases generally appeared to be higher during ictal/peri-ictal administration, were transient, and were not dose dependent.7
**Seizure interval was measured as the time between VALTOCO doses using seizure diary data from the open-label safety study. Seventy-six of the 163 patients (47%) who received ≥1 dose of VALTOCO in the study had ≥1 seizure interval in each of Periods 1, 2, 3, and 4 and were included in the consistent cohort for Periods 1-4. Retreatments, defined as second doses given within 24 hours of the first doses, were eliminated from the analysis.8
See study designs


VALTOCO optimizes diazepam for nasal delivery
Diazepam
Diazepam has a relatively long half-life vs other benzodiazepines, which may contribute to sustained drug levels. Among benzodiazepines, it is one of the most highly lipophilic, leading to rapid distribution to the brain.1,12,13
Intravail®
Intravail® increases drug absorption across the nasal mucosa by increasing permeability of cell membranes and temporarily loosening tight junctions between cells, allowing the drug to pass through.14-16
Vitamin E
Vitamin E increases the solubility of diazepam to allow adequate concentration in a small volume of nasal spray.7
Diazepam rapidly enters the bloodstream after VALTOCO administration 1,14,††
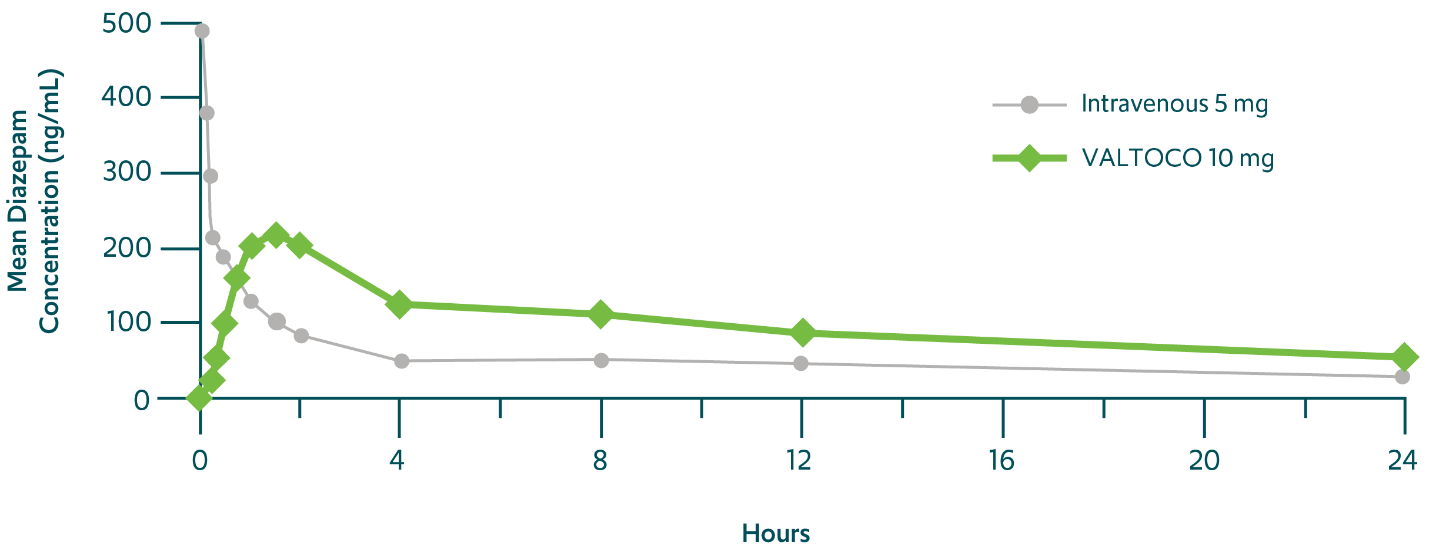
Even up to 24 hours, VALTOCO can reduce the likelihood of further seizures in a seizure episode3
††Phase 1, randomized, open-label, crossover bioavailability and pharmacokinetic study in 24 healthy volunteers.14
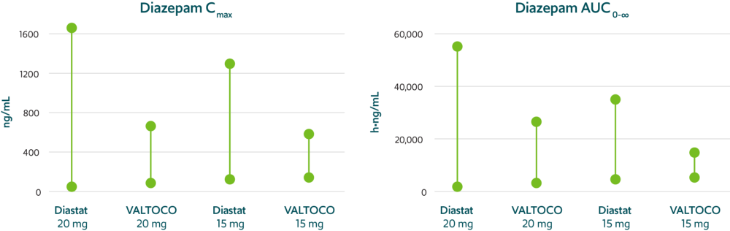
Predictable pharmacokinetics with low interpatient and intrapatient variability1,15,17,‡‡,§§
VALTOCO demonstrated 2- to 4-fold less pharmacokinetic variability than Diastat® (diazepam rectal gel) regardless of patient weight1,15,17,§§
Pharmacokinetic variability profiles of VALTOCO and Diastat17,§§
AUC, area under the curve; Cmax, maximum serum concentration.
90% CI
- VALTOCO pharmacokinetics were not notably altered when administered during seizures vs under normal conditions7,‖‖
‡‡In adults.
§§Phase 1, randomized, open-label crossover bioavailability and safety study in 48 healthy volunteers.17
‖‖Phase 1, open-label, crossover pharmacokinetic and safety study in 57 adult patients with epilepsy.7
VALTOCO was deemed clinically superior in administration to Diastat® (diazepam rectal gel) by the US Food and Drug Administration (FDA)18,##
The intranasal route of administration of VALTOCO provides a major contribution to patient care over the rectal route of administration by providing a significantly improved ease of use.18
Because of this, the FDA has determined that VALTOCO deserves orphan drug exclusivity.18
##Granted at time of original approval in 2020 in patients 6+ years of age.
VALTOCO was strongly preferred in a patient survey4,***,†††
***Results compared with diazepam rectal gel.
†††From an exit survey (n=66 patients, n=84 caregivers) of a phase 3, open-label, repeat-dose safety study of VALTOCO in patients 6 to 65 years of age.4
90% said it was “extremely easy” or “very easy” to train others to administer VALTOCO
87% felt more comfortable being treated in public with VALTOCO than with diazepam rectal gel
84% said they would prefer using VALTOCO as their seizure rescue exclusively in the future
79% were very comfortable doing activities outside the home with VALTOCO available
Improvements in seizure worry and social functioning19,‡‡‡
PATIENTS 18+ YEARS OF AGE WITH EPILEPSY
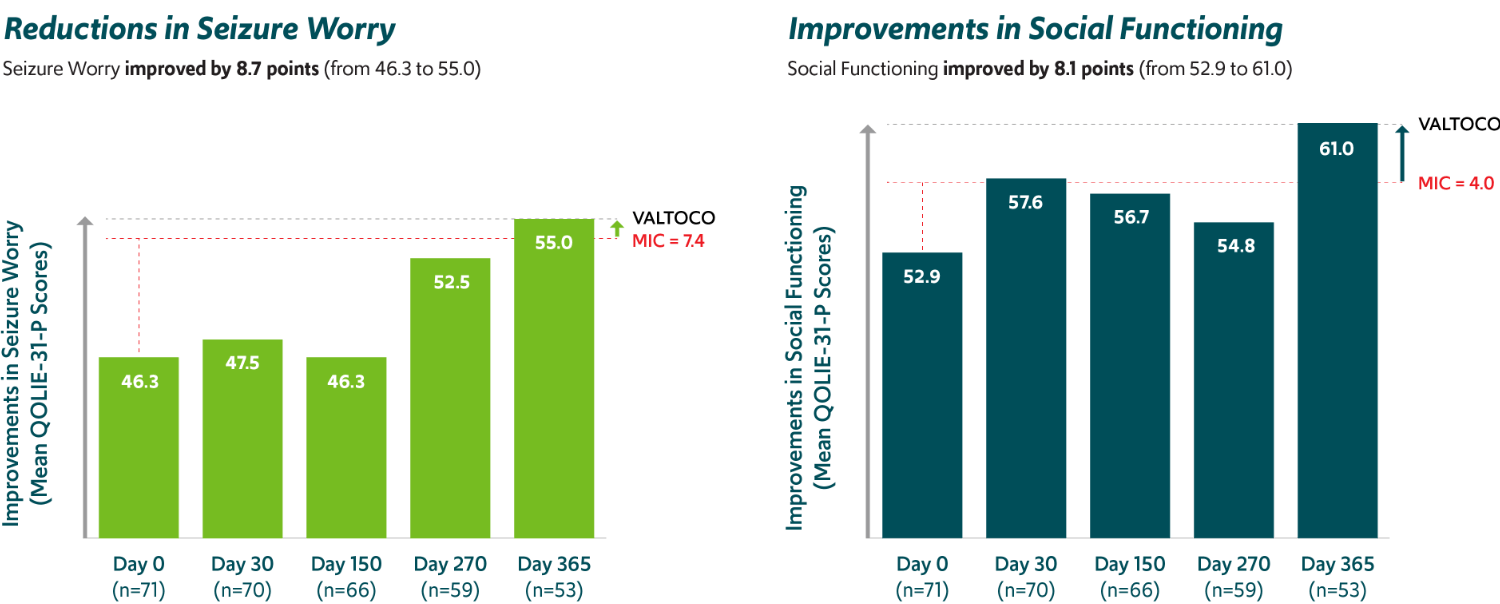
Mean total QOLIE-31-P scores increased from day 0 (n=71) to day 365 (n=53) by 5.2 points (from 57.3 to 62.5), which also exceeded the MIC threshold of 5.19.
MIC, minimally important change; QOLIE-31-P, Quality of Life in Epilepsy—Problems.
‡‡‡Exploratory analysis of a 12-month, open-label, repeat-dose safety study in patients 6 to 65 years of age; the study did not have prespecified efficacy endpoints.5

Help your patients get to know VALTOCO
Get all the resources you need so you can educate your patients about VALTOCO.
