Helpful VALTOCO resources
These resources can help set patients and their care partners up for success with VALTOCO.

Learn how to use VALTOCO
To help you and your patients practice how to administer VALTOCO, the Demo Kit contains everything you need to know to use the device with confidence. The Demo Kit also includes the following:
- Demo device with Instructions for Use
- myNEURELIS® personalized support and savings information
- Prescribing Information and Medication Guide
Resources for you and your patients
Download resources individually below or download the entire resource kit.

Patient Brochure
This guide can help patients understand how to use VALTOCO, what individualized dosing looks like, and the programs available to them.
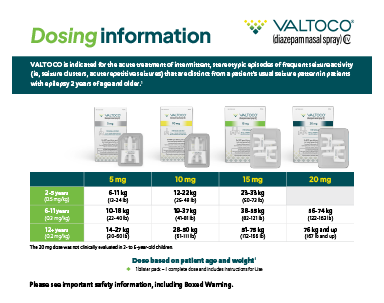
Dosing Card
Learn about the available VALTOCO dosing options for pediatric and adult patients.


Prescription Form
VALTOCO is available to order through US retail pharmacies. To send your prescriptions through Maxor Specialty Pharmacy, use this form.

Patient Tip Sheet
This guide can help your patients learn how to get their VALTOCO prescription via Maxor Specialty Pharmacy.

Office Tip Sheet
Help ensure your patients receive the VALTOCO you’ve prescribed by following the simple steps laid out in this document.
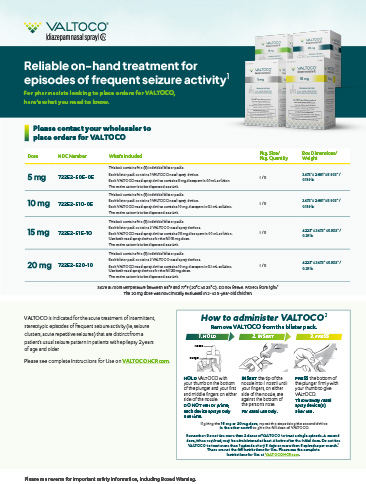
Trade Sheet
Print out this trade sheet and keep it handy for when it’s time to place orders of VALTOCO for patients.

Instructions for Use (IFU)
Instructions that your patients, their family members, care partners, and others who may need to give VALTOCO should read before using.
Resources for school nurses
Download resources individually below or download the entire resource kit.

School Nurse Frequently Asked Questions
Review commonly asked questions about VALTOCO so that you’ll be well versed on this seizure rescue medication.

VALTOCO Information Sheet for School Nurses
Get important information about having VALTOCO on hand at school so that you’ll be ready to help students when they need it most.

Advocate for VALTOCO: A Letter to Parents of Students
Support parents and care partners of students with episodes of frequent seizures by advocating for the compassionate choice. This letter may help.

School Seizure Action Plan
Ensure that a current seizure action plan is on file for students with epilepsy. Review it annually with the student, their parents or care partners, and their healthcare provider. The Epilepsy Foundation has seizure action plan templates like this one available in multiple languages.
